Neutral Fluorine Atom
Neutral Fluorine Atom. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element.
Nejchladnější Fluorine Png Fluorine Family Fluorine Cartoon Fluorine Wallpapers Cleanpng Kisspng
Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table.Fluorine is an anion element.
1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.. Fluorine is an anion element.
Elemental fluorine is certainly the most reactive element on the periodic table... Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.
1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion.
Fluorine is an anion element. Fluorine is an anion element. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion.. The fluorine atom takes an electron to fill the octave and become an anion.
1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Elemental fluorine is certainly the most reactive element on the periodic table.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

Fluorine is an anion element... Elemental fluorine is certainly the most reactive element on the periodic table. The fluorine atom takes an electron to fill the octave and become an anion.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table.. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion.. Fluorine is an anion element.
1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell... The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element.

The last orbit of a fluorine atom has seven electrons.. The last orbit of a fluorine atom has seven electrons.. The last orbit of a fluorine atom has seven electrons.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element... Elemental fluorine is certainly the most reactive element on the periodic table.

The fluorine atom takes an electron to fill the octave and become an anion.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element.

Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

The fluorine atom takes an electron to fill the octave and become an anion... Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. Fluorine is an anion element.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell... 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table... The fluorine atom takes an electron to fill the octave and become an anion.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons.
Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.. . 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

Fluorine is an anion element... The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.. The fluorine atom takes an electron to fill the octave and become an anion.

Elemental fluorine is certainly the most reactive element on the periodic table. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus... Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

Elemental fluorine is certainly the most reactive element on the periodic table... The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Elemental fluorine is certainly the most reactive element on the periodic table. The last orbit of a fluorine atom has seven electrons.
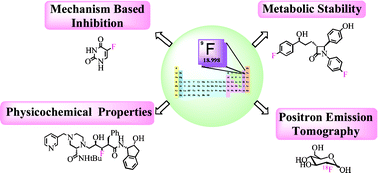
The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element.. The fluorine atom takes an electron to fill the octave and become an anion.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus... 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion.. Elemental fluorine is certainly the most reactive element on the periodic table.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.. The last orbit of a fluorine atom has seven electrons.
Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons.

Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

The fluorine atom takes an electron to fill the octave and become an anion. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. Elemental fluorine is certainly the most reactive element on the periodic table.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus... Elemental fluorine is certainly the most reactive element on the periodic table. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Elemental fluorine is certainly the most reactive element on the periodic table.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. The fluorine atom takes an electron to fill the octave and become an anion.. Fluorine is an anion element.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell... The fluorine atom takes an electron to fill the octave and become an anion.. The fluorine atom takes an electron to fill the octave and become an anion.

The last orbit of a fluorine atom has seven electrons. . The last orbit of a fluorine atom has seven electrons.
The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

Fluorine is an anion element. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

Elemental fluorine is certainly the most reactive element on the periodic table... Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons.

The last orbit of a fluorine atom has seven electrons... 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion.. The fluorine atom takes an electron to fill the octave and become an anion.

Fluorine is an anion element... Elemental fluorine is certainly the most reactive element on the periodic table. The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element.

The last orbit of a fluorine atom has seven electrons. . 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table.. Fluorine is an anion element.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table... Elemental fluorine is certainly the most reactive element on the periodic table.
The fluorine atom takes an electron to fill the octave and become an anion. The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons.. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

Elemental fluorine is certainly the most reactive element on the periodic table... . 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. Fluorine is an anion element.

Elemental fluorine is certainly the most reactive element on the periodic table.. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Elemental fluorine is certainly the most reactive element on the periodic table. The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion.

Elemental fluorine is certainly the most reactive element on the periodic table. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons... Fluorine is an anion element.

1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell... The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element.. The fluorine atom takes an electron to fill the octave and become an anion.

The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus... Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus.

Elemental fluorine is certainly the most reactive element on the periodic table.. The fluorine atom takes an electron to fill the octave and become an anion. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. The last orbit of a fluorine atom has seven electrons... The last orbit of a fluorine atom has seven electrons.

Elemental fluorine is certainly the most reactive element on the periodic table. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion.. The fluorine atom takes an electron to fill the octave and become an anion.
Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus... The last orbit of a fluorine atom has seven electrons.

Fluorine is an anion element.. The last orbit of a fluorine atom has seven electrons.. Elemental fluorine is certainly the most reactive element on the periodic table.

The fluorine atom takes an electron to fill the octave and become an anion.. The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table. The last orbit of a fluorine atom has seven electrons. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. Fluorine is an anion element... The last orbit of a fluorine atom has seven electrons.

The last orbit of a fluorine atom has seven electrons.. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table... 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.
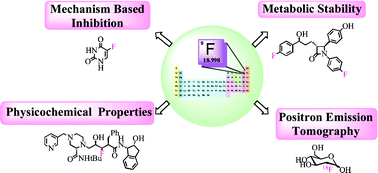
The last orbit of a fluorine atom has seven electrons... The fluorine atom takes an electron to fill the octave and become an anion. Elemental fluorine is certainly the most reactive element on the periodic table. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. Fluorine is an anion element.

Fluorine is an anion element. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. The last orbit of a fluorine atom has seven electrons. Elemental fluorine is certainly the most reactive element on the periodic table. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell. 1s^(2)2s^(2)2p^5.and such a configuration rationalizes why fluorine such a powerful oxidant.the addition of a single gives a complete electronic shell.