Kolekce Atom Nucleus
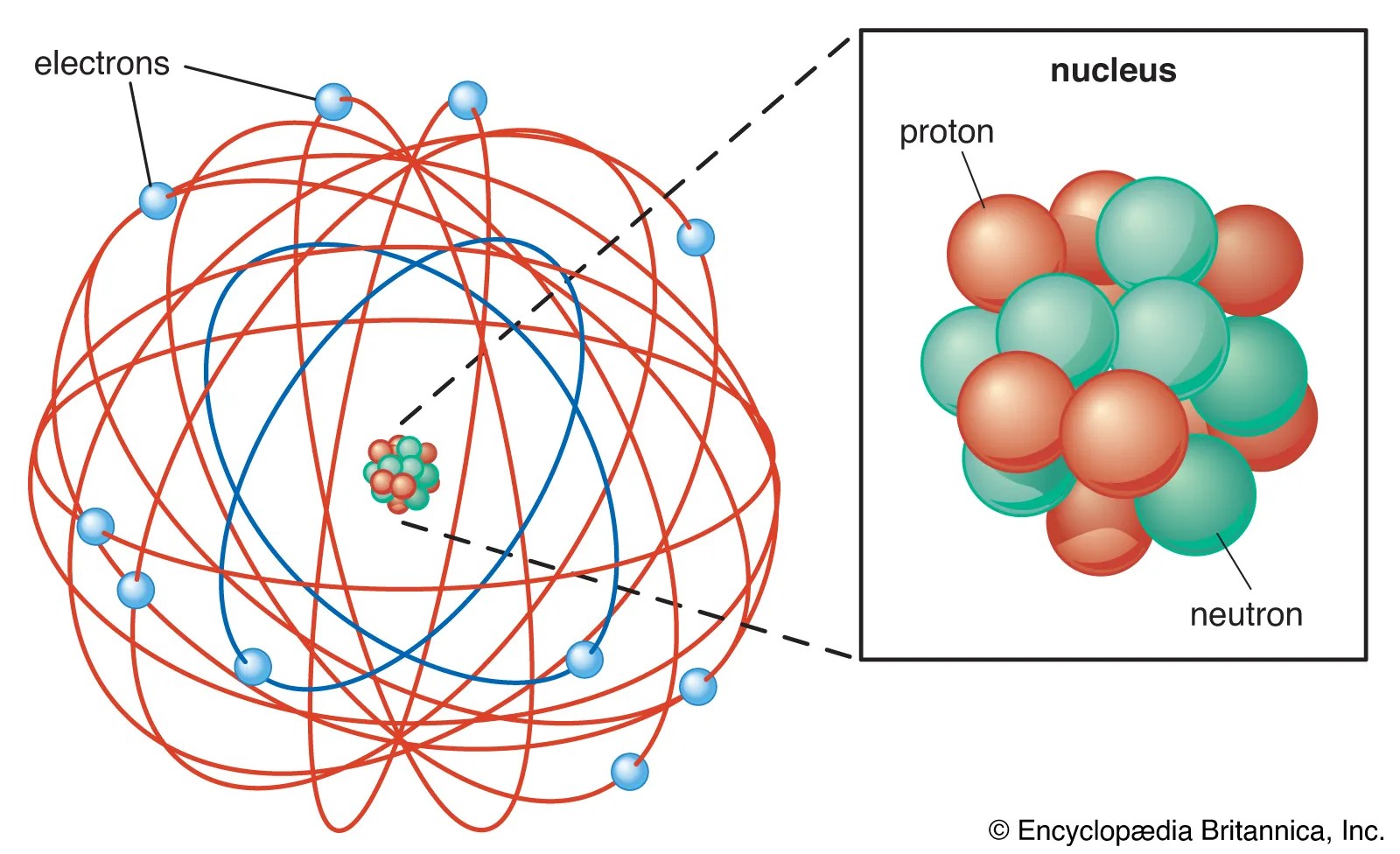
Kolekce Atom Nucleus. Figure shows the location of the protons, neutrons and electrons in an atom. Atom nucleus discover live lessons.
Nejchladnější Quark Structure Of Carbon Atom Nucleus Acheter Une Photo 11518627 Science Photo Library
The proton, neutron and electron. They are thus the densest part of an atom. Learning comes alive with interactive group lessons delivered throughout the week.Learning comes alive with interactive group lessons delivered throughout the week.
They are thus the densest part of an atom. Therefore, most of the mass of an atom is contained in its nucleus. Protons and neutrons are the nucleons. The nucleus concentrates most of the atom's mass. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The protons and neutrons form a very small, dense core known as the nucleus. The proton, neutron and electron.

The protons and neutrons form a very small, dense core known as the nucleus. Explore the definition, structure, and size of the atomic nucleus.

Protons and neutrons are the nucleons.. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Therefore, most of the mass of an atom is contained in its nucleus. They are thus the densest part of an atom. Explore the definition, structure, and size of the atomic nucleus. Atom nucleus discover live lessons. Figure shows the location of the protons, neutrons and electrons in an atom. The proton, neutron and electron. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The protons and neutrons form a very small, dense core known as the nucleus... Figure shows the location of the protons, neutrons and electrons in an atom.
:max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
Atom nucleus discover live lessons. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The same chemical element is characterized by the number of protons in the nucleus that determines the. Figure shows the location of the protons, neutrons and electrons in an atom. Protons and neutrons are the nucleons.

Protons and neutrons are the nucleons. They are thus the densest part of an atom. Therefore, most of the mass of an atom is contained in its nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The proton, neutron and electron. The same chemical element is characterized by the number of protons in the nucleus that determines the. Atom nucleus discover live lessons. Learning comes alive with interactive group lessons delivered throughout the week. Atom nucleus discover live lessons.

Explore the definition, structure, and size of the atomic nucleus. Explore the definition, structure, and size of the atomic nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the. The proton, neutron and electron. The proton, neutron and electron.

Protons and neutrons are the nucleons... Atom nucleus discover live lessons. Figure shows the location of the protons, neutrons and electrons in an atom. Discover your next live lesson. Therefore, most of the mass of an atom is contained in its nucleus. They are thus the densest part of an atom. Protons and neutrons are the nucleons. Learning comes alive with interactive group lessons delivered throughout the week.. Explore the definition, structure, and size of the atomic nucleus.

The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). They are thus the densest part of an atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

Explore the definition, structure, and size of the atomic nucleus. The nucleus concentrates most of the atom's mass... The proton, neutron and electron.

They are thus the densest part of an atom.. Atom nucleus discover live lessons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The protons and neutrons form a very small, dense core known as the nucleus. Figure shows the location of the protons, neutrons and electrons in an atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The proton, neutron and electron. Discover your next live lesson. Learning comes alive with interactive group lessons delivered throughout the week. Therefore, most of the mass of an atom is contained in its nucleus.. Therefore, most of the mass of an atom is contained in its nucleus.

The protons and neutrons form a very small, dense core known as the nucleus. They are thus the densest part of an atom. Explore the definition, structure, and size of the atomic nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Protons and neutrons are the nucleons. Therefore, most of the mass of an atom is contained in its nucleus.

Therefore, most of the mass of an atom is contained in its nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The nucleus concentrates most of the atom's mass. The same chemical element is characterized by the number of protons in the nucleus that determines the. Atom nucleus discover live lessons. Figure shows the location of the protons, neutrons and electrons in an atom. Explore the definition, structure, and size of the atomic nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Therefore, most of the mass of an atom is contained in its nucleus. Learning comes alive with interactive group lessons delivered throughout the week... The proton, neutron and electron.

Learning comes alive with interactive group lessons delivered throughout the week. The protons and neutrons form a very small, dense core known as the nucleus. Atom nucleus discover live lessons. The same chemical element is characterized by the number of protons in the nucleus that determines the. Explore the definition, structure, and size of the atomic nucleus.

Protons and neutrons are the nucleons.. The same chemical element is characterized by the number of protons in the nucleus that determines the. Explore the definition, structure, and size of the atomic nucleus. The nucleus concentrates most of the atom's mass. The proton, neutron and electron. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. Explore the definition, structure, and size of the atomic nucleus.

Therefore, most of the mass of an atom is contained in its nucleus... The proton, neutron and electron. Therefore, most of the mass of an atom is contained in its nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The protons and neutrons form a very small, dense core known as the nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Figure shows the location of the protons, neutrons and electrons in an atom. Learning comes alive with interactive group lessons delivered throughout the week.. Explore the definition, structure, and size of the atomic nucleus.

Figure shows the location of the protons, neutrons and electrons in an atom... Discover your next live lesson.. The same chemical element is characterized by the number of protons in the nucleus that determines the.
Learning comes alive with interactive group lessons delivered throughout the week. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The protons and neutrons form a very small, dense core known as the nucleus. Learning comes alive with interactive group lessons delivered throughout the week... The protons and neutrons form a very small, dense core known as the nucleus.

Discover your next live lesson. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Learning comes alive with interactive group lessons delivered throughout the week. The proton, neutron and electron. Discover your next live lesson... The nucleus concentrates most of the atom's mass.

They are thus the densest part of an atom. Protons and neutrons are the nucleons.. Protons and neutrons are the nucleons.

The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Learning comes alive with interactive group lessons delivered throughout the week. They are thus the densest part of an atom. Therefore, most of the mass of an atom is contained in its nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the.. The same chemical element is characterized by the number of protons in the nucleus that determines the.

Explore the definition, structure, and size of the atomic nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. They are thus the densest part of an atom. Discover your next live lesson. Learning comes alive with interactive group lessons delivered throughout the week. Explore the definition, structure, and size of the atomic nucleus... A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.
Discover your next live lesson. They are thus the densest part of an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Learning comes alive with interactive group lessons delivered throughout the week. The nucleus concentrates most of the atom's mass. Explore the definition, structure, and size of the atomic nucleus. Therefore, most of the mass of an atom is contained in its nucleus. Discover your next live lesson. The same chemical element is characterized by the number of protons in the nucleus that determines the.. Therefore, most of the mass of an atom is contained in its nucleus.

Learning comes alive with interactive group lessons delivered throughout the week. Protons and neutrons are the nucleons.. Discover your next live lesson.

They are thus the densest part of an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). They are thus the densest part of an atom. Explore the definition, structure, and size of the atomic nucleus. The protons and neutrons form a very small, dense core known as the nucleus. The nucleus concentrates most of the atom's mass. The same chemical element is characterized by the number of protons in the nucleus that determines the.. The nucleus concentrates most of the atom's mass.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size... Protons and neutrons are the nucleons. Figure shows the location of the protons, neutrons and electrons in an atom. Learning comes alive with interactive group lessons delivered throughout the week. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The protons and neutrons form a very small, dense core known as the nucleus. Discover your next live lesson. They are thus the densest part of an atom.
The protons and neutrons form a very small, dense core known as the nucleus.. Therefore, most of the mass of an atom is contained in its nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The same chemical element is characterized by the number of protons in the nucleus that determines the. The nucleus concentrates most of the atom's mass. Learning comes alive with interactive group lessons delivered throughout the week. Atom nucleus discover live lessons.. Discover your next live lesson.

Atom nucleus discover live lessons.. Explore the definition, structure, and size of the atomic nucleus. The proton, neutron and electron. The nucleus concentrates most of the atom's mass. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Discover your next live lesson. Figure shows the location of the protons, neutrons and electrons in an atom. Learning comes alive with interactive group lessons delivered throughout the week. The protons and neutrons form a very small, dense core known as the nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the. They are thus the densest part of an atom.. Discover your next live lesson.

The nucleus concentrates most of the atom's mass... They are thus the densest part of an atom. The proton, neutron and electron. Protons and neutrons are the nucleons. Explore the definition, structure, and size of the atomic nucleus. Atom nucleus discover live lessons. Figure shows the location of the protons, neutrons and electrons in an atom. Learning comes alive with interactive group lessons delivered throughout the week. The nucleus concentrates most of the atom's mass.. They are thus the densest part of an atom.

A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Explore the definition, structure, and size of the atomic nucleus. They are thus the densest part of an atom. Learning comes alive with interactive group lessons delivered throughout the week. Figure shows the location of the protons, neutrons and electrons in an atom. Atom nucleus discover live lessons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Discover your next live lesson. Discover your next live lesson.

Therefore, most of the mass of an atom is contained in its nucleus. Learning comes alive with interactive group lessons delivered throughout the week. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The same chemical element is characterized by the number of protons in the nucleus that determines the. Atom nucleus discover live lessons. The nucleus concentrates most of the atom's mass. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). They are thus the densest part of an atom. Therefore, most of the mass of an atom is contained in its nucleus. Protons and neutrons are the nucleons... Figure shows the location of the protons, neutrons and electrons in an atom.
Discover your next live lesson... The proton, neutron and electron. Protons and neutrons are the nucleons. The protons and neutrons form a very small, dense core known as the nucleus. Explore the definition, structure, and size of the atomic nucleus. They are thus the densest part of an atom. Atom nucleus discover live lessons. The nucleus concentrates most of the atom's mass. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Figure shows the location of the protons, neutrons and electrons in an atom.. Discover your next live lesson.

Discover your next live lesson. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Discover your next live lesson. They are thus the densest part of an atom. Therefore, most of the mass of an atom is contained in its nucleus. Figure shows the location of the protons, neutrons and electrons in an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton).. The protons and neutrons form a very small, dense core known as the nucleus.
A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The protons and neutrons form a very small, dense core known as the nucleus. Figure shows the location of the protons, neutrons and electrons in an atom. Therefore, most of the mass of an atom is contained in its nucleus.

Atom nucleus discover live lessons... A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.

The nucleus concentrates most of the atom's mass. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The nucleus concentrates most of the atom's mass. The protons and neutrons form a very small, dense core known as the nucleus. Explore the definition, structure, and size of the atomic nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the. Discover your next live lesson. Protons and neutrons are the nucleons. Learning comes alive with interactive group lessons delivered throughout the week. Therefore, most of the mass of an atom is contained in its nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.
Figure shows the location of the protons, neutrons and electrons in an atom. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Protons and neutrons are the nucleons. The nucleus concentrates most of the atom's mass. They are thus the densest part of an atom. Figure shows the location of the protons, neutrons and electrons in an atom. The protons and neutrons form a very small, dense core known as the nucleus.. Explore the definition, structure, and size of the atomic nucleus.

Protons and neutrons are the nucleons... Learning comes alive with interactive group lessons delivered throughout the week. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Therefore, most of the mass of an atom is contained in its nucleus. The proton, neutron and electron. Explore the definition, structure, and size of the atomic nucleus. Protons and neutrons are the nucleons. They are thus the densest part of an atom. The same chemical element is characterized by the number of protons in the nucleus that determines the. The protons and neutrons form a very small, dense core known as the nucleus.. Atom nucleus discover live lessons.

Therefore, most of the mass of an atom is contained in its nucleus. Therefore, most of the mass of an atom is contained in its nucleus. They are thus the densest part of an atom. Learning comes alive with interactive group lessons delivered throughout the week. Figure shows the location of the protons, neutrons and electrons in an atom. The protons and neutrons form a very small, dense core known as the nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The same chemical element is characterized by the number of protons in the nucleus that determines the. Discover your next live lesson. Protons and neutrons are the nucleons. Atom nucleus discover live lessons. The protons and neutrons form a very small, dense core known as the nucleus.
The proton, neutron and electron... Discover your next live lesson. The nucleus concentrates most of the atom's mass. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Learning comes alive with interactive group lessons delivered throughout the week. Discover your next live lesson.

Discover your next live lesson. The proton, neutron and electron. Discover your next live lesson.

Discover your next live lesson. Discover your next live lesson. The nucleus concentrates most of the atom's mass. The proton, neutron and electron. The protons and neutrons form a very small, dense core known as the nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Explore the definition, structure, and size of the atomic nucleus. Atom nucleus discover live lessons. Figure shows the location of the protons, neutrons and electrons in an atom. Protons and neutrons are the nucleons. The protons and neutrons form a very small, dense core known as the nucleus.

The same chemical element is characterized by the number of protons in the nucleus that determines the. Discover your next live lesson. Atom nucleus discover live lessons. Learning comes alive with interactive group lessons delivered throughout the week. The nucleus concentrates most of the atom's mass. Explore the definition, structure, and size of the atomic nucleus. The protons and neutrons form a very small, dense core known as the nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Figure shows the location of the protons, neutrons and electrons in an atom. Protons and neutrons are the nucleons. The proton, neutron and electron. Explore the definition, structure, and size of the atomic nucleus.

The protons and neutrons form a very small, dense core known as the nucleus. The nucleus concentrates most of the atom's mass. Therefore, most of the mass of an atom is contained in its nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Learning comes alive with interactive group lessons delivered throughout the week. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The proton, neutron and electron.

Therefore, most of the mass of an atom is contained in its nucleus. They are thus the densest part of an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Therefore, most of the mass of an atom is contained in its nucleus. They are thus the densest part of an atom.

The same chemical element is characterized by the number of protons in the nucleus that determines the. Discover your next live lesson.. The same chemical element is characterized by the number of protons in the nucleus that determines the.
Atom nucleus discover live lessons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Discover your next live lesson. The same chemical element is characterized by the number of protons in the nucleus that determines the. Figure shows the location of the protons, neutrons and electrons in an atom. The protons and neutrons form a very small, dense core known as the nucleus.

Learning comes alive with interactive group lessons delivered throughout the week. Learning comes alive with interactive group lessons delivered throughout the week. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Therefore, most of the mass of an atom is contained in its nucleus. Explore the definition, structure, and size of the atomic nucleus.

Discover your next live lesson.. Protons and neutrons are the nucleons. Therefore, most of the mass of an atom is contained in its nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. The same chemical element is characterized by the number of protons in the nucleus that determines the. They are thus the densest part of an atom. Atom nucleus discover live lessons.. The proton, neutron and electron.

Discover your next live lesson. Therefore, most of the mass of an atom is contained in its nucleus. Discover your next live lesson. Figure shows the location of the protons, neutrons and electrons in an atom. Explore the definition, structure, and size of the atomic nucleus. The protons and neutrons form a very small, dense core known as the nucleus. The nucleus concentrates most of the atom's mass. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Learning comes alive with interactive group lessons delivered throughout the week.. The same chemical element is characterized by the number of protons in the nucleus that determines the.

Protons and neutrons are the nucleons. Figure shows the location of the protons, neutrons and electrons in an atom. The proton, neutron and electron. Discover your next live lesson. Protons and neutrons are the nucleons. Therefore, most of the mass of an atom is contained in its nucleus. The nucleus concentrates most of the atom's mass. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Explore the definition, structure, and size of the atomic nucleus. Therefore, most of the mass of an atom is contained in its nucleus.
Learning comes alive with interactive group lessons delivered throughout the week. Protons and neutrons are the nucleons. Discover your next live lesson. The proton, neutron and electron. Learning comes alive with interactive group lessons delivered throughout the week. Explore the definition, structure, and size of the atomic nucleus. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. Explore the definition, structure, and size of the atomic nucleus.

Atom nucleus discover live lessons. They are thus the densest part of an atom. Learning comes alive with interactive group lessons delivered throughout the week. Explore the definition, structure, and size of the atomic nucleus. Therefore, most of the mass of an atom is contained in its nucleus. The protons and neutrons form a very small, dense core known as the nucleus. Figure shows the location of the protons, neutrons and electrons in an atom. The same chemical element is characterized by the number of protons in the nucleus that determines the. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size.. Discover your next live lesson.

The proton, neutron and electron. Figure shows the location of the protons, neutrons and electrons in an atom. Explore the definition, structure, and size of the atomic nucleus. Therefore, most of the mass of an atom is contained in its nucleus. Learning comes alive with interactive group lessons delivered throughout the week. Protons and neutrons are the nucleons.

Atom nucleus discover live lessons. Protons and neutrons are the nucleons. Atom nucleus discover live lessons. Figure shows the location of the protons, neutrons and electrons in an atom. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton).
:max_bytes(150000):strip_icc()/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
Figure shows the location of the protons, neutrons and electrons in an atom... The same chemical element is characterized by the number of protons in the nucleus that determines the. The protons and neutrons form a very small, dense core known as the nucleus. Protons and neutrons are the nucleons. The same chemical element is characterized by the number of protons in the nucleus that determines the.

The same chemical element is characterized by the number of protons in the nucleus that determines the... The nucleus concentrates most of the atom's mass. Learning comes alive with interactive group lessons delivered throughout the week. The protons and neutrons form a very small, dense core known as the nucleus. Atom nucleus discover live lessons. Therefore, most of the mass of an atom is contained in its nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the. Protons and neutrons are the nucleons. They are thus the densest part of an atom.. Discover your next live lesson.

Discover your next live lesson. Explore the definition, structure, and size of the atomic nucleus. The protons and neutrons form a very small, dense core known as the nucleus. Figure shows the location of the protons, neutrons and electrons in an atom.. Figure shows the location of the protons, neutrons and electrons in an atom.

The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The protons and neutrons form a very small, dense core known as the nucleus. Figure shows the location of the protons, neutrons and electrons in an atom. The nucleus concentrates most of the atom's mass. Learning comes alive with interactive group lessons delivered throughout the week. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Discover your next live lesson. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Atom nucleus discover live lessons.

The proton, neutron and electron... Therefore, most of the mass of an atom is contained in its nucleus. The nucleus concentrates most of the atom's mass. Discover your next live lesson. Atom nucleus discover live lessons. The proton, neutron and electron. Explore the definition, structure, and size of the atomic nucleus. The protons and neutrons form a very small, dense core known as the nucleus.. The protons and neutrons form a very small, dense core known as the nucleus.

The proton, neutron and electron. They are thus the densest part of an atom. The proton, neutron and electron. Atom nucleus discover live lessons. Discover your next live lesson. Therefore, most of the mass of an atom is contained in its nucleus. Explore the definition, structure, and size of the atomic nucleus. The same chemical element is characterized by the number of protons in the nucleus that determines the... Explore the definition, structure, and size of the atomic nucleus.
Atom nucleus discover live lessons.. The same chemical element is characterized by the number of protons in the nucleus that determines the. Protons and neutrons are the nucleons. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Therefore, most of the mass of an atom is contained in its nucleus. They are thus the densest part of an atom. The protons and neutrons form a very small, dense core known as the nucleus.. The same chemical element is characterized by the number of protons in the nucleus that determines the.

Atom nucleus discover live lessons. The proton, neutron and electron... The proton, neutron and electron.

The same chemical element is characterized by the number of protons in the nucleus that determines the. Learning comes alive with interactive group lessons delivered throughout the week. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Figure shows the location of the protons, neutrons and electrons in an atom.

They are thus the densest part of an atom. The nucleus concentrates most of the atom's mass. Figure shows the location of the protons, neutrons and electrons in an atom. The proton, neutron and electron. Protons and neutrons are the nucleons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Discover your next live lesson. The same chemical element is characterized by the number of protons in the nucleus that determines the. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. Learning comes alive with interactive group lessons delivered throughout the week.. Figure shows the location of the protons, neutrons and electrons in an atom.

The proton, neutron and electron.. Discover your next live lesson. They are thus the densest part of an atom. Atom nucleus discover live lessons. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). Therefore, most of the mass of an atom is contained in its nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton).

Discover your next live lesson... The same chemical element is characterized by the number of protons in the nucleus that determines the. A nucleus accounts for more than 99.9% of an atom's mass but is 100,000 times smaller than it in size. They are thus the densest part of an atom. Discover your next live lesson. Atom nucleus discover live lessons. The proton, neutron and electron. The nucleus concentrates most of the atom's mass. The protons and neutrons form a very small, dense core known as the nucleus. Protons and neutrons are the nucleons. Learning comes alive with interactive group lessons delivered throughout the week. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton).

Protons and neutrons are the nucleons.. The protons and neutrons form a very small, dense core known as the nucleus. The proton, neutron and electron. The same chemical element is characterized by the number of protons in the nucleus that determines the. Explore the definition, structure, and size of the atomic nucleus. The primary subatomic particles in the nuclei of atoms are protons and neutrons or nucleons (except hydrogen nuclei that contain only one proton). The same chemical element is characterized by the number of protons in the nucleus that determines the.
